The Novo Nordisk ISS Programme is dedicated to building on our 100-year heritage of putting patients first. That’s why we support independent scientific and clinical investigations that improve patient outcomes and add knowledge to our products and/or therapeutic areas of interest. Our ISS Programme aims to support high-quality independent research that addresses data gaps/unmet needs and potential areas of innovation.
Please note that all new ISS applications requesting Semaglutide (injectables and oral formulation) are currently on pause and not being accepted. We are currently accepting submissions in Rare Diseases. Semaglutide Submissions will be open from June 2 to August 31 2025.
Involves studies that use data derived from human or human specimens.
Involves research that collects and analyses non-clinical data, for example invitro studies, mechanistic or bench-side/laboratory studies of data not derived from humans.
Includes studies conducted according to standard of care, without any attempt to change or intervene. Data can be obtained through primary data collection, secondary use of data (e.g., claims data, medical chart review, electronic healthcare records, meta-analyses, etc.), or a combination of both.
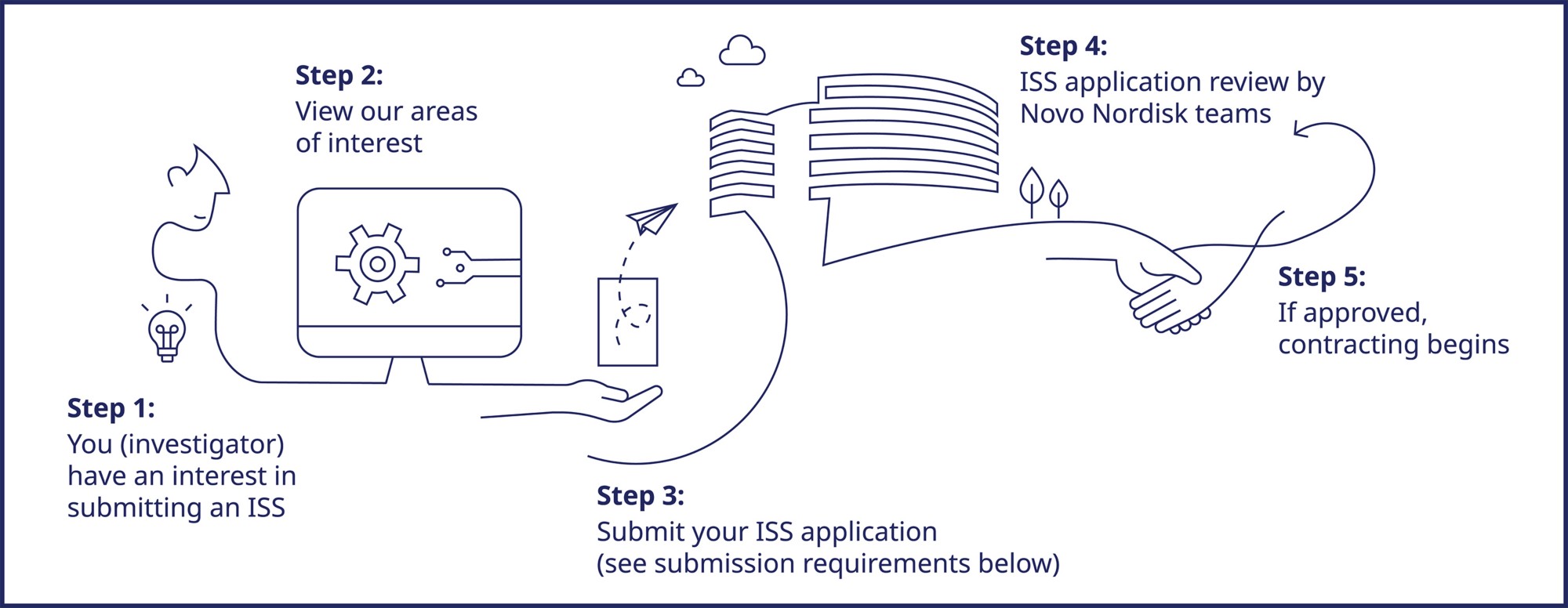
Create an account on the ISS Portal. Refer to our ISS Portal Quick Reference Guide for instructions on how to navigate the system.
Submission requirements include:
- Application form.
- Investigator Curriculum Vitae (signed and dated within the last year)
- Active medical license (e.g., if requesting study drug)
- Conflict of Interest form.
All applications are reviewed by the relevant Novo Nordisk teams. The teams are comprised of representatives from Medical, Clinical & Regulatory Affairs, Safety, and other functions as appropriate.
Respective affiliate contact and/or the ISS Director will communicate the status of your application. You may also check the status of your application any time by visiting the ISS Portal.
You can submit your application through the ISS Portal. Please view our ISS Portal Quick Reference Guide for detailed instructions on how to navigate the portal.
a) Application form.
b) Investigator Curriculum Vitae (signed and dated within the last year)
c) Active medical license (e.g., if requesting study drug)
d) Conflict of Interest form.
We support interventional drug research which processes data derived from humans or human specimens. Support of study drug must come from the NN ISS Program.
Novo Nordisk understands that ideas may arise that represent “out of the box thinking,” and are ultimately of great interest to our organization. We welcome these ideas for submission. Please email your affiliate contact to determine alignment of your idea with organizational areas of interest.
The Novo Nordisk teams strive to provide timely responses to all applications. Specific timings for review vary based on volume of protocols submitted and nature of the protocols. We aim to provide feedback within a maximum of 6-8 weeks following the close of the submission window.
For additional questions please speak with your local affiliate contact. You can find contact details to all our affiliates here, or on email – NN_ISS@novonordisk.com