Employees and external stakeholders can report suspected misconduct in a secure and confidential manner through the Novo Nordisk Compliance Hotline.
When you have a concern about a possible breach of regulation or internal policies, the right thing is to raise your question or concern to relevant people.
You may use these links for reporting to the Compliance Hotline:
Compliance Hotline reporting portal
Compliance Hotline reporting portal - Iran (Please use this link if you're located in Iran).
Business Ethics misconduct, which could be a conflict of interest, corruption including payment of bribery or facilitation payments, unethical donations, gifts and entertainment to business partners, public officials or healthcare professionals given by Novo Nordisk employees or third parties acting on behalf of Novo Nordisk, Human Rights abuses or protection of Privacy and Personal Data.
Accounting issues & Fraud, such as theft and embezzlement of Novo Nordisk assets and funds, vendor fraud, acceptance of kickbacks, accounting manipulation, breaches in internal accounting controls and auditing matters.
Quality misconduct impacting GxP related processes which are not being addressed in the regular Quality management system.
Breaches of antitrust or fair-trading legislations, which could be related to misuse of dominant position on a market.
Breaches of environmental legislations, such as possible illegal disposal of waste.
Novo Nordisk Way misconduct, including serious unfair treatment of an individual employee or broader management misbehaviour.
You may also report serious misconduct related to Espionage, Sabotage, Information Security Violations and other breaches that otherwise relate to serious offence or other serious matters.
Ombudsman
Individual cases concerning unfair treatment of a single
employee will in most cases be handled by Ombudsman falling outside of
the investigational process and whistle-blower set-up. You can report
cases to Ombudsman via this link: Ombudsman (for
Novo Nordisk employees only)
Whenever you suspect misconduct, we encourage you to report it. However, you should not use the reporting system to make false accusations or to spread unfounded rumours.
Concerns about, but not limited to disagreements with colleagues or practical complaints (equipment malfunctions, office environment, holiday issues) or other issues covered by collective bargaining agreements with unions, or other complaints about e.g. smoking or dress codes may not be reported on the Compliance Hotline.
Further, note that the Novo Nordisk Compliance Hotline should not be used for reporting of product complaints, falsified products or side effects. You should report these issues via these links:
How to report your concern
It is possible to
report a concern to the compliance hotline by telephone or online.
For both options, please click on the link below for reporting and
further guidance.
Compliance Hotline reporting portal
Compliance
Hotline reporting portal - Iran (Please use this link if you're
located in Iran).
If you prefer making the report
face-to-face, you may do so either to your immediate manager or by
contacting local Legal and Compliance. You may also indicate your
request for meeting in person via phone or online without providing
further details of your concern.
You can select among multiple language options, and instructions for calling. Phone numbers are provided on the website.
Please notice that when reporting a case by phone, a short waiting time can be expected before an agent is available to take your call.
Acknowledgement of receipt
The receipt of your report will be acknowledged within 7 days from the day of your reporting.
Follow-up on your concern
When you report a case
you will get an Access Number and will be asked to create a
password. This way you will be able to communicate with Compliance
Hotline staff in an anonymous way.
We encourage you to follow-up on your report to verify your reporting, check the status of your concern, provide additional information or to check whether we have additional questions to better investigate your report.
Feedback
We will provide you feedback to you report within a reasonable timeframe in general not exceeding 3 months.
Reporting to authorities
If you reside in EU, you may - alternatively to reporting to Novo Nordisk - report a concern to the local external reporting channel available in your country. The external reporting channel is managed by a competent authority in your country permitting reporting similar to reporting internally to Novo Nordisk by phone, in writing or by physical meeting.
For information of which competent authority is managing the external reporting channel, you are referred to the Novo Nordisk Compliance Hotline webpage of your country, see section on Local Reporting Rights and Obligations below. In Denmark, the external whistle-blower reporting channel is the Danish Data Protection Agency.
You are encouraged to report concerns to Novo Nordisk first.
When reporting a case, it will be received by the Audit Committee Secretariat monitored by the Audit Committee, which is part of the Novo Nordisk Board of Directors.
For reports in scope of the investigational process and whistleblower set-up and where an investigation is assessed necessary, an investigation of your concern will be started up including establishment of the relevant investigation team.
The investigation process is outlined below.
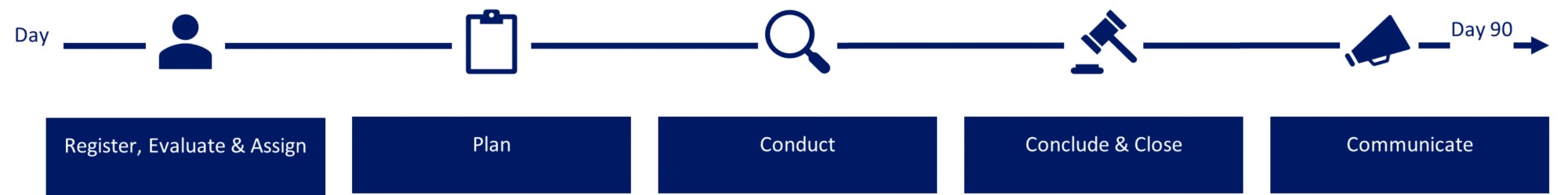
Novo Nordisk will not tolerate discrimination or retaliation against reporting persons or persons and companies assisting in making the report who in good faith make a report or persons participating in an investigation. Retaliation occurs when an adverse or credible threat of an adverse action is taken against an employee who in good faith makes a report or participate in an investigation simply because he or she did so.
Examples of retaliation include, but are not limited to:
- Disciplinary action against a reporting employee
- Denying a promotion to a reporting employee
- Issuing a poor performance evaluation of a reporting employee
- Preventing or attempting to prevent persons from making a report
- Cancellation of license or permit
- Blacklisting
This procedure does not prohibit adverse action taken against employees for legitimate, non-retaliatory reasons, even if that employee is engaged in protected activity, as long as the adverse action is not directly connected to the reporting or investigation participation.
Whether you provide your name or chose to stay anonymous, Novo Nordisk will diligently ensure your protection to the extent possible and that your identity is not revealed.
If you identify yourself, it increases our ability to investigate the report and maintain the contact for more information and provide feedback to you during the investigation and protect you against potential retaliation.
Your name and identity will be kept confidential and will in general not be shared with persons outside the investigation team. Should sharing of your identity under the circumstances be necessary, we will seek your consent to do so, unless it is specifically permitted not to involve you.
The Investigation Team is working under confidentiality obligations and is trained to safeguard whistleblower reports and investigative information.
If you reside in EU, information of possible specific rights and obligations under national law in your country, please visit the Novo Nordisk Compliance Hotline webpage of your country.
Reports received through the Compliance Hotline may include personal data. Novo Nordisk will process personal data in accordance with the applicable personal data protection rules.